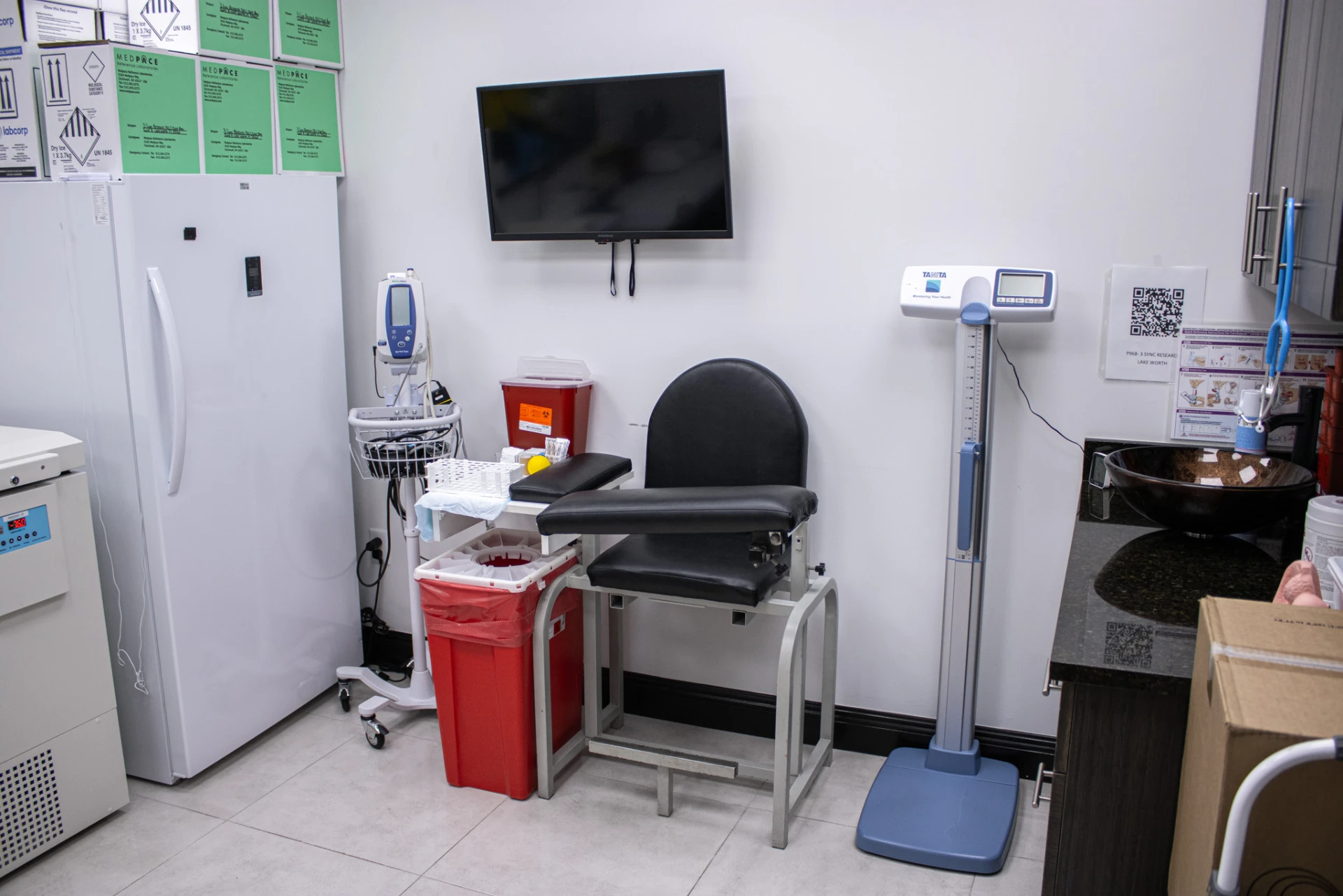
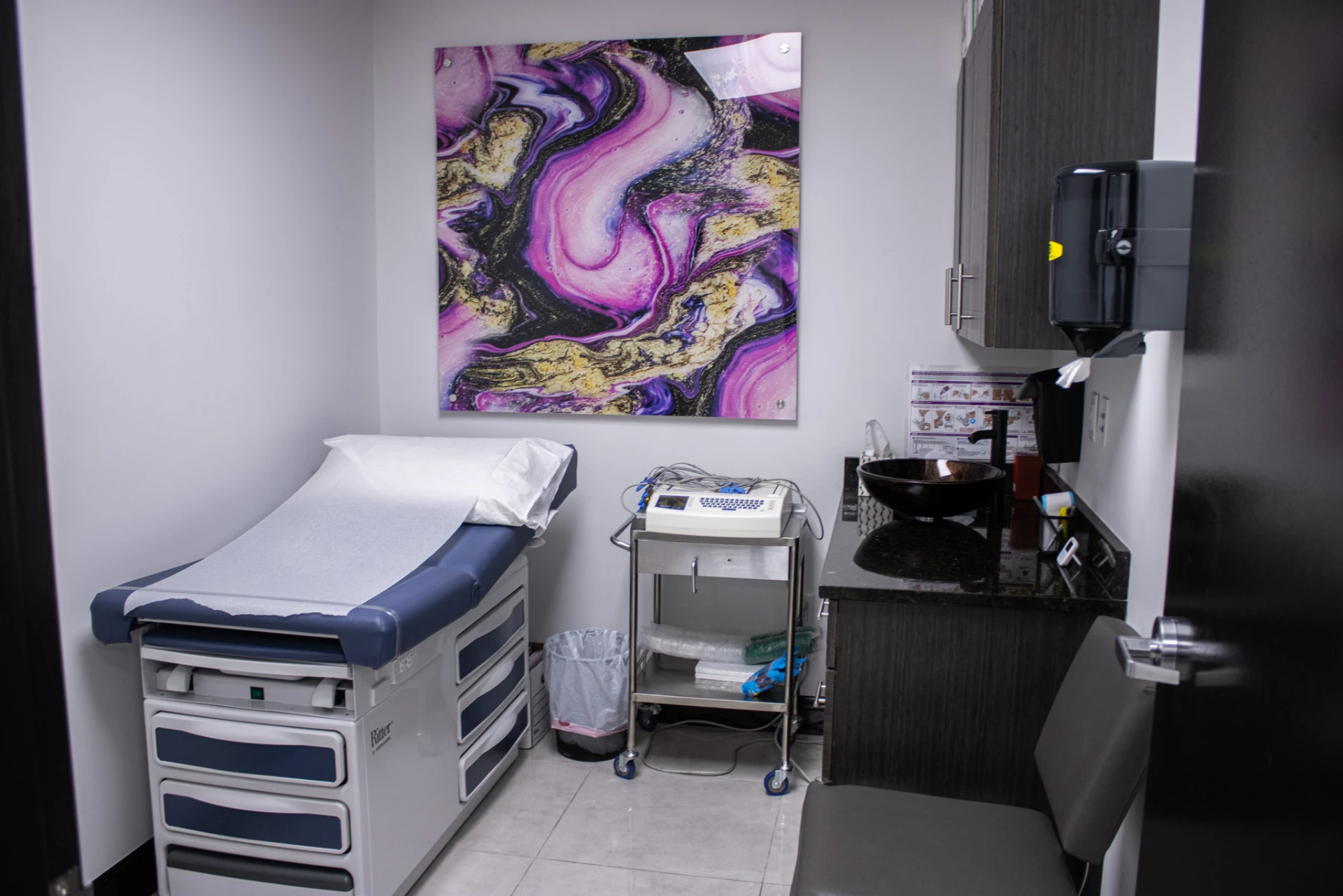
CUTTING EDGE CLINICAL RESEARCH
Together, we can shift the paradigm of clinical research, offering opportunities to those that previously have not had the chance to realize the benefits of new drugs and devices!
MISSION
Soma Medical Center, P.A. firmly believes in the purpose of research studies, and clinical trials, which seek to determine the effectiveness of new treatments. Our goal is to enable doctors to study safe & effective treatments, accurate diagnoses, and even preventative medical care. Our aim is to bridge the gap between research opportunities and doctors by providing the necessary time, resources, and support to explore new molecules, devices, and treatment options across diverse therapeutic elds.
Soma Medical Center, P.A. is dedicated to clinical research to ensure all our clinical trial partners, participants, sponsors, and investigators can rely on us for unparalleled service and exceptional performance.
Our mission is centered around democratizing clinical research, striving to bring its benefits to as many people as possible. By doing so, we aim to break down barriers, improve healthcare outcomes, and empower doctors from all backgrounds to contribute to cutting-edge medical advancements.
Improve Your Quality of Life
being a part of a research study means taking a proactive role in your healthcare, which could improve or even save your life.
provide additional care
Studies usually offer diagnodstic tests and even a full physical before you get started, a great way to get an update on your overall health.
learn more about your condition
When you participate in a study, you’ll get insights into your specific health condition from highly trained specialists.
Access to leading research doctors
During your study you’ll receive one-on-one medical care from board-certified physicians and a skilled research team.
provide additional income for your time and travel
We will compensate participants, with the amount varying, depending on the study’s length and the type of treatment you receive.
access to innovative new treatments.
Through your study, you’ll have access to new medicines and treatments at no cost.
About Clinical Research
We partner with BSYNC Research A Multi-Site, Multi-Specialty, Wholly-Owned Research Company. Their Corporate Headquarters are located in Sunrise, Florida with additional locations in Dade, Broward, and Palm Beach Counties.

Karen Tveten
Business hours
-
Sunday
Closed Monday
08:00 AM - 05:00 PMTuesday
08:00 AM - 05:00 PMWednesday
08:00 AM - 05:00 PMThursday
08:00 AM - 05:00 PMFriday
08:00 AM - 05:00 PM-
Saturday
Closed
Active Clinical Studies

COPD (Smoker’s) Study
Study Compensation: up to $2350COPD (Smoker’s) Study
Study Compensation: up to $2350
The P1-COPD-04-INT study is evaluating how switching to a product called the Tobacco Heating System (THS) may impact symptoms and the disease progression of early chronic obstructive pulmonary disease (COPD) in smokers.
- Are between 40 and 65 years of age.
- Have been smoking for at least 10 years.
- Have been smoking a minimum of 10 cigarettes a day for at least the last year.
- Have not used other tobacco and nicotine products apart from cigarettes on a daily basis over the past year.
- Have experienced cough and sputum (symptoms of chronic bronchitis) over the past year OR have been diagnosed with mild-to-moderate COPD.
- Are not pregnant, if you are a female.
- THS and sticks, if you decide to enroll into this group.
- Close monitoring of your health through repeated, detailed check-ups, and medical supervision and care.
- Smoking cessation advice (provided to all study subjects).
- Behavioral support if you chose to stop smoking or to switch to THS.
- Nicotine replacement therapy if you decide to stop smoking and would like to be provided with such therapy.
- Reimbursement for study participation may be available.
Diabetic Neuropathy Study
Study Compensation: up to: $1100Diabetic Neuropathy Study
Study Compensation: up to: $1100
A Phase 2 Study to Evaluate the Safety and Efficacy of RTA 901 in Patients with Diabetic Peripheral Neuropathic Pain.
- Adult male and female subjects ≥ 18 years of age upon study consent.
- Diagnosis of type 1 diabetes mellitus or type 2 diabetes mellitus at least 1 year prior to Screening.
- Clinical diagnosis of DPNP defined as symptomatic distal symmetric polyneuropathy(secondary to diabetes) in the lower extremities, which may include symptoms of pain that is burning, lancinating, tingling, or shooting (electric shock–like). Pain in the lower extremities may occur with paresthesia or dysesthesia (unpleasant sensations of burning). Neuropathic pain may be accompanied by an exaggerated response to painful stimuli (hyperalgesia)
- Has neuropathy from a cause other than type 1 diabetes mellitus or type 2 diabetes mellitus.
- Has a condition other than DPNP that could confound the assessment of pain (eg, fibromyalgia or regional pain caused by lumbar or cervical compression);
- Hemoglobin A1C > 11% at Screening.
- Diabetic foot ulceration or infection within 90 days prior to Screening.
- Has had more than 1 episode of ketoacidosis or hyperosmolar state requiring hospitalization within 90 days prior to Screening.
- Has had more than 3 episodes of hypoglycemia requiring medical assistance within 90 days prior to Screening.
- Any acute or chronic medical condition, or concurrent therapy (pharmaceutical or otherwise) which, in the opinion of the Investigator could potentially adversely impact subject safety, response to study drug, or interfere with study assessments.
- Clinically significant and severe ophthalmologic disease, including but not limited to retinopathy, or visual field impairment which, in the opinion of the Investigator could potentially preclude enrollment in the study.
- Serum aminotransferase (alanine aminotransferase or aspartate aminotransferase) levels > 1.5× the upper limit of normal at Screening.
- Has HIV or active hepatitis B or C virus infection.
Lung Cancer Study
Compensation: $100(1 visit)Lung Cancer Study
Compensation: $100(1 visit)
Early Detection of Lung Cancer.
- Diagnostic performance of blood test following up imaging after positive low dose CT Screening for cancer of the lung.
- Adult male and female patient aged 50 to 80.